
The light intensity, over a narrow frequency range, gets increased because of the emission by the material.

In comparison, when photons from some of the hot material are observed in the presence of a broad spectrum from a cold source, a bright emission spectrum line is also formed. The intensity of light over a narrow frequency range can be reduced because of absorption by the material and re-emission in random directions. An absorption line can be formed when photons from a broad and hot spectrum source pass via cold material. The type of line observed will depend on the type of material and its temperature relative to the other source of emission. Then it will be re-emitted spontaneously at the same frequency as the cascade or the original, where the sum of the emitted photon energies will be equal to the energy of the absorbed photon (assuming that the system returns to the original state).Ī spectral line can be observed either as an absorption line or an emission spline. When the photon has up to the right amount of energy (connected to its frequency) to allow a change in the system's energy state (in the case of an atom, this is generally an electron changing orbitals), the photon can be absorbed. The association between a quantum system (usually electrons, but at times, atomic nuclei or molecules) and a single photon is the product of spectral lines.

These "fingerprints" are compared with the previously collected "fingerprints" of molecules and atoms and are therefore used to identify (which would otherwise be impossible) the molecular and atomic components of planets and stars. Spectral lines are often used in the identification of molecules and atoms.
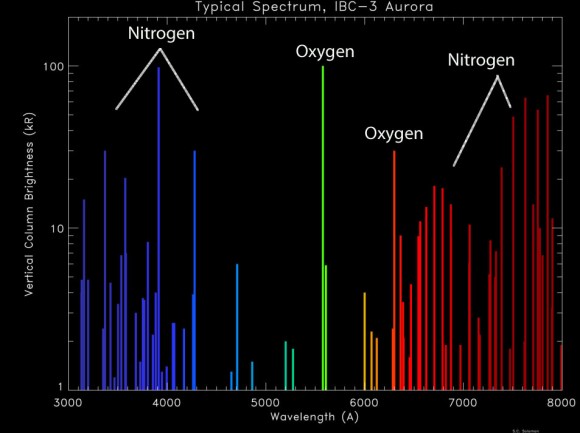
A spectral line is defined as a dark or bright line in an otherwise continuous and uniform spectrum, resulting from light’s absorption or emission in a narrow frequency range, compared with the nearby frequencies.


 0 kommentar(er)
0 kommentar(er)
